Ch3 Ch2 Ch Ch3 2
khabri
Sep 15, 2025 · 7 min read
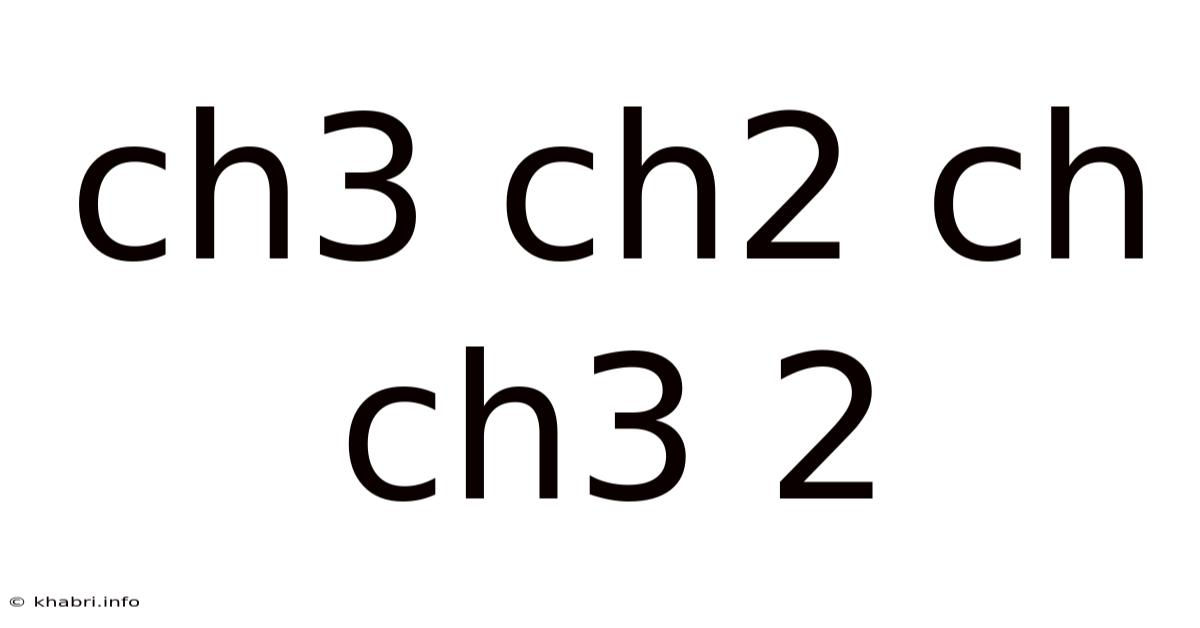
Table of Contents
Understanding the Mysterious "CH3CH2CHCH3": Isomers, Properties, and Reactions of Butene
This article delves into the fascinating world of organic chemistry, specifically focusing on the compound represented by the formula CH₃CH₂CHCH₃. This seemingly simple formula actually represents several different molecules, known as isomers, each with unique properties and reactivity. We'll explore the different isomers of butene, their structural characteristics, physical and chemical properties, and common reactions. Understanding this seemingly simple molecule unlocks a deeper understanding of isomerism and the complexities of organic chemistry.
Introduction to Butene Isomers
The formula CH₃CH₂CHCH₃ represents a family of unsaturated hydrocarbons called butenes (or but-enes). These are alkenes, meaning they contain a carbon-carbon double bond (C=C). The presence of this double bond introduces isomerism, creating several variations of the molecule with different arrangements of atoms. The key to understanding these isomers lies in the position of the double bond and the possibility of cis-trans (or E-Z) isomerism.
Let's break down the possible isomers:
-
1-Butene (But-1-ene): The double bond is located between the first and second carbon atoms. The structure is CH₂=CHCH₂CH₃.
-
2-Butene (But-2-ene): The double bond is located between the second and third carbon atoms. This isomer exhibits cis-trans isomerism:
-
cis-2-Butene (or Z-2-Butene): The two methyl (CH₃) groups are on the same side of the double bond.
-
trans-2-Butene (or E-2-Butene): The two methyl (CH₃) groups are on opposite sides of the double bond.
-
Therefore, the formula CH₃CH₂CHCH₃, without specifying the location of the double bond, is ambiguous and refers to a mixture of 1-butene and the cis and trans isomers of 2-butene.
Structural Characteristics and Bonding
Understanding the structure of each isomer is crucial to predicting its properties. The carbon atoms in each butene molecule are sp² hybridized, meaning each carbon atom involved in the double bond has three sp² hybrid orbitals and one unhybridized p orbital. The sp² hybrid orbitals form sigma (σ) bonds with neighboring carbon and hydrogen atoms, while the unhybridized p orbitals overlap laterally to form a pi (π) bond. This π bond is weaker than the σ bond and is responsible for the reactivity of alkenes.
The cis-trans isomerism in 2-butene arises from the restricted rotation around the carbon-carbon double bond. The π bond prevents free rotation, resulting in distinct spatial arrangements of the substituents around the double bond. cis-2-Butene has a slightly higher boiling point than trans-2-butene due to its higher dipole moment caused by the closer proximity of the methyl groups.
Physical Properties of Butene Isomers
The physical properties of the butene isomers, like boiling point, density, and solubility, vary slightly depending on their structure.
-
Boiling Point: The boiling points generally increase with increasing molecular weight and the degree of branching. trans-2-Butene generally has a slightly lower boiling point than cis-2-Butene, and 1-butene has a boiling point between the two 2-butene isomers.
-
Density: All butene isomers are less dense than water, meaning they are immiscible (do not mix) with water.
-
Solubility: Butenes are nonpolar molecules and are therefore soluble in nonpolar solvents such as hydrocarbons, but insoluble in polar solvents like water.
The precise values of these physical properties can vary slightly depending on the purity of the sample and the measuring conditions.
Chemical Properties and Reactions of Butenes
The double bond in butenes makes them highly reactive towards a range of chemical reagents. Several crucial reactions are worth exploring:
-
Addition Reactions: The most common reaction of alkenes is the addition of other molecules across the double bond. This typically involves breaking the π bond and forming two new σ bonds.
-
Hydrogenation: Adding hydrogen (H₂) in the presence of a metal catalyst (e.g., platinum, palladium, or nickel) converts butene to butane (CH₃CH₂CH₂CH₃), a saturated hydrocarbon.
-
Halogenation: Adding halogens (e.g., chlorine (Cl₂), bromine (Br₂)) across the double bond forms dihaloalkanes. For example, 1-butene reacts with bromine to form 1,2-dibromobutane.
-
Hydrohalogenation: Adding hydrogen halides (e.g., HCl, HBr) across the double bond forms haloalkanes. Markovnikov's rule predicts the regioselectivity of this reaction, meaning the hydrogen atom adds to the carbon atom that already has more hydrogen atoms.
-
Hydration: Adding water (H₂O) in the presence of an acid catalyst (e.g., sulfuric acid) converts butene to an alcohol (butanol). Again, Markovnikov's rule governs the regioselectivity.
-
-
Polymerization: Butenes can undergo polymerization, forming long chains of repeating butene units. This is an important industrial process used to produce polybutene, a polymer used in various applications.
-
Oxidation: Butenes can be oxidized by strong oxidizing agents, leading to the formation of various products depending on the oxidizing agent and reaction conditions. For example, potassium permanganate (KMnO₄) can cleave the double bond, resulting in the formation of carboxylic acids.
Spectroscopic Characterization of Butenes
Different spectroscopic techniques are essential for identifying and characterizing the various butene isomers. These techniques provide crucial information about the molecular structure and bonding.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: ¹H NMR spectroscopy provides information about the number and types of hydrogen atoms in the molecule. The chemical shifts and coupling patterns can distinguish between the different butene isomers. For instance, the cis and trans isomers of 2-butene exhibit different coupling constants between the methyl protons and the vinylic protons due to the difference in their spatial arrangement.
-
Infrared (IR) Spectroscopy: IR spectroscopy detects the presence of functional groups by analyzing the vibrational frequencies of bonds. The C=C stretching frequency is characteristic of alkenes and provides evidence for the presence of a double bond. Differences in the IR spectra of the isomers can help distinguish between them.
-
Mass Spectrometry (MS): MS provides information about the molecular weight and fragmentation pattern of the molecule. The fragmentation pattern can sometimes help distinguish between isomers.
These spectroscopic techniques, used in conjunction, provide a powerful tool for the complete identification and characterization of butene isomers.
Applications of Butenes
Butenes, particularly 1-butene, have various important industrial applications:
-
Production of Polybutene: 1-Butene is a major monomer used in the production of polybutene, a thermoplastic polymer with applications in adhesives, sealants, and lubricants.
-
Production of Isobutene: 1-Butene can be isomerized to isobutene, which is used in the production of methyl tert-butyl ether (MTBE), a gasoline additive.
-
Production of Butanol: Butenes are used in the production of various butanol isomers, which find applications as solvents and intermediates in the chemical industry.
-
Synthetic Rubber Production: Butenes can be used as comonomers in the production of synthetic rubbers.
Frequently Asked Questions (FAQ)
Q: What is the difference between 1-butene and 2-butene?
A: The difference lies in the position of the double bond. In 1-butene, the double bond is located between the first and second carbon atoms, while in 2-butene, it's between the second and third carbon atoms. This difference in structure leads to variations in reactivity and physical properties.
Q: How can I distinguish between cis-2-butene and trans-2-butene?
A: The most reliable method is using spectroscopic techniques like NMR spectroscopy. The coupling constants between the methyl protons and the vinylic protons differ significantly between the two isomers due to their different spatial arrangements. Gas chromatography can also be used to separate and identify the isomers based on their boiling points.
Q: Are butenes toxic?
A: Butenes are flammable and can be harmful if inhaled in high concentrations. They are considered relatively low toxicity compared to many other organic compounds, but appropriate safety precautions should always be followed when handling them.
Q: What are the environmental impacts of butenes?
A: Butenes are greenhouse gases, contributing to climate change. Their release into the atmosphere should be minimized through proper industrial practices. They are also involved in the formation of ground-level ozone, a major component of smog.
Conclusion
The seemingly simple chemical formula CH₃CH₂CHCH₃ actually represents a family of isomers, each with its unique properties and applications. Understanding the nuances of butene isomerism – the position of the double bond and cis-trans isomerism – is crucial for comprehending their reactivity, physical properties, and industrial uses. From addition reactions to polymerization, butenes play a significant role in the chemical industry, and their continued study remains essential for advancements in materials science and chemical engineering. The detailed examination of their structural characteristics, spectroscopic identification techniques, and diverse applications highlights the complexity and fascinating nature of organic chemistry. This deep dive into the world of butenes hopefully provides a comprehensive understanding of this important class of organic compounds.
Latest Posts
Latest Posts
-
Business Organized As A Corporation
Sep 15, 2025
-
Full Sentence Outline Format Example
Sep 15, 2025
-
4 Ethyl 2 2 Dimethyloctane
Sep 15, 2025
-
Storage Encompasses How Information Is
Sep 15, 2025
-
Which Statement Best Describes Hormones
Sep 15, 2025
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 Ch Ch3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.