4 Ethyl 2 2 Dimethyloctane
khabri
Sep 15, 2025 · 6 min read
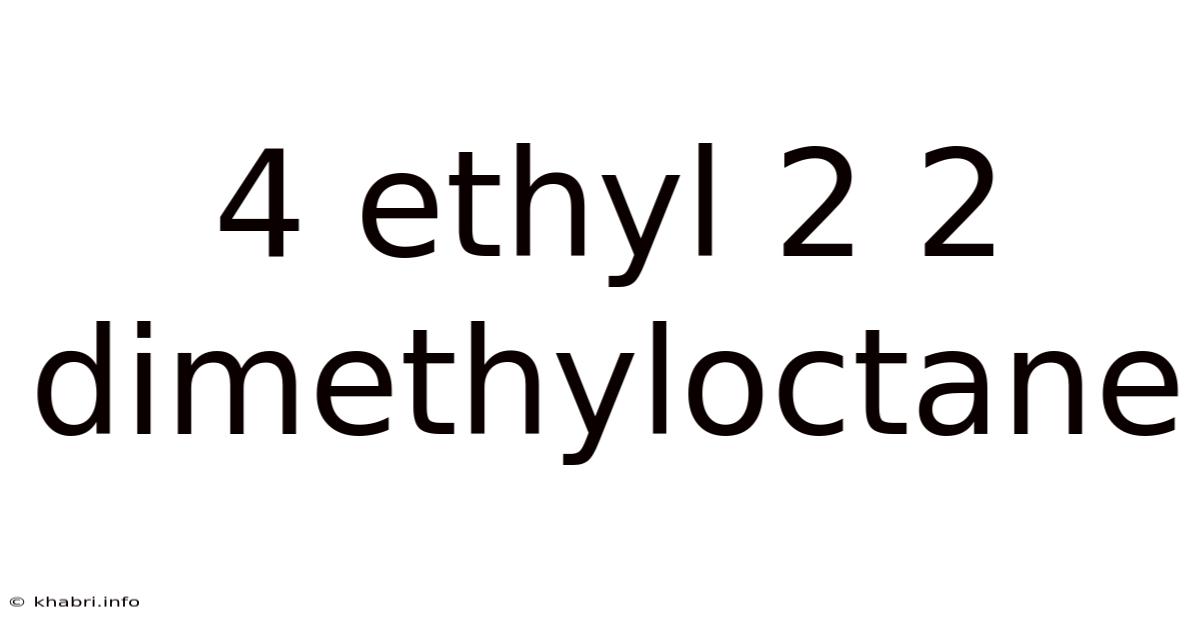
Table of Contents
Unveiling the Mysteries of 4-Ethyl-2,2-Dimethyloctane: A Deep Dive into its Structure, Properties, and Applications
4-Ethyl-2,2-dimethyloctane, a seemingly complex name, represents a specific organic compound with interesting properties. Understanding its structure, synthesis, properties, and potential applications requires a detailed examination. This article aims to provide a comprehensive overview of 4-ethyl-2,2-dimethyloctane, suitable for both students and researchers interested in organic chemistry and its industrial applications. We will explore its chemical characteristics, potential uses, and delve into the specifics of its molecular structure.
Understanding the Molecular Structure of 4-Ethyl-2,2-Dimethyloctane
At its core, 4-ethyl-2,2-dimethyloctane is an alkane, a type of saturated hydrocarbon characterized by single carbon-carbon bonds. The name itself reveals its structure:
-
Octane: This indicates an eight-carbon chain as the parent hydrocarbon. Imagine a straight chain of eight carbon atoms.
-
2,2-Dimethyl: This signifies two methyl groups (–CH₃) attached to the second carbon atom in the chain. This creates branching, deviating from a linear structure.
-
4-Ethyl: An ethyl group (–CH₂CH₃) is attached to the fourth carbon atom in the chain. This further adds to the branching complexity.
Therefore, the molecule's full structure is a branched eight-carbon chain with two methyl groups on the second carbon and an ethyl group on the fourth carbon. This specific arrangement significantly influences its properties. Visualizing this structure with the help of a molecular model kit or a chemical drawing program is highly recommended for a complete understanding.
Key Structural Features and their Implications:
-
Branched Structure: The branching significantly impacts the molecule's boiling point and melting point compared to a straight-chain octane isomer. Branched alkanes generally have lower boiling and melting points due to reduced surface area and weaker intermolecular forces (van der Waals forces).
-
Isomerism: 4-Ethyl-2,2-dimethyloctane is just one of many possible isomers of C₁₀H₂₂ (decane). The different arrangements of atoms lead to variations in physical and chemical properties. Understanding isomerism is crucial in organic chemistry.
-
Aliphatic Nature: The compound is aliphatic, meaning its carbon atoms are arranged in an open chain rather than a ring structure (like in aromatic compounds).
Physical and Chemical Properties: A Detailed Analysis
Several physical and chemical properties define 4-ethyl-2,2-dimethyloctane. These properties are critical for determining its potential applications and handling safety measures.
-
State of Matter: At room temperature and standard pressure, 4-ethyl-2,2-dimethyloctane exists as a colorless liquid.
-
Boiling Point: Due to its branched structure, its boiling point is lower than that of its linear isomer, n-decane. Precise boiling point values require careful experimental determination under controlled conditions, and may vary slightly based on purity.
-
Melting Point: Similar to the boiling point, the melting point is relatively low, reflecting the weaker intermolecular forces in the branched structure.
-
Density: The density is typically less than that of water, making it less dense and immiscible with water.
-
Solubility: Being a non-polar hydrocarbon, 4-ethyl-2,2-dimethyloctane is insoluble in water but soluble in many organic solvents like hexane, benzene, and toluene. Its solubility in different solvents depends on the polarity of the solvent.
-
Flammability: Like most hydrocarbons, 4-ethyl-2,2-dimethyloctane is highly flammable. Appropriate safety precautions should be taken during handling and storage to prevent fires.
-
Reactivity: As a saturated hydrocarbon, it exhibits relatively low reactivity. It does not readily undergo reactions like addition or oxidation under normal conditions. However, under specific conditions (high temperatures, presence of catalysts), it can undergo combustion, cracking, or halogenation.
Synthesis of 4-Ethyl-2,2-Dimethyloctane
The synthesis of 4-ethyl-2,2-dimethyloctane isn't typically a straightforward, single-step process. It likely involves multiple steps and careful reaction conditions. Common strategies in organic synthesis to build branched alkanes include:
-
Grignard Reactions: These reactions are powerful tools in organic chemistry for forming carbon-carbon bonds. A strategically designed Grignard reagent could be used to build the branched carbon skeleton.
-
Wurtz Coupling: This method involves the reaction of two alkyl halides with sodium metal to form a new carbon-carbon bond. Carefully selected alkyl halides are essential to produce the desired branching pattern.
-
Alkylation Reactions: This involves the addition of an alkyl group to an existing molecule. Selective alkylation reactions can be used to introduce the ethyl and methyl branches.
The exact synthesis route would depend on the availability of starting materials and the desired yield and purity of the final product. Optimization of reaction conditions, such as temperature, solvent, and catalyst, is crucial for efficient synthesis.
Potential Applications and Industrial Relevance
While 4-ethyl-2,2-dimethyloctane may not be a widely known chemical like benzene or ethanol, its properties suggest several potential applications:
-
Solvent: Its non-polar nature and solubility in various organic solvents make it potentially useful as a solvent in certain industrial processes, particularly where a non-polar, high-boiling point solvent is required. Specific applications would require thorough testing and consideration of its flammability.
-
Component in Fuel Blends: Due to its hydrocarbon nature, it could potentially be a component in fuel blends. However, detailed analysis of its combustion properties and environmental impact would be essential before any industrial application in this area.
-
Chemical Intermediate: It could serve as a chemical intermediate in the synthesis of other more complex molecules, although specific examples require further research and development.
Safety Precautions and Handling
Given its flammability and the potential health hazards associated with hydrocarbon exposure, careful handling and safety measures are crucial when working with 4-ethyl-2,2-dimethyloctane:
-
Flammability: Keep away from open flames and ignition sources. Use in well-ventilated areas to prevent the buildup of flammable vapors.
-
Inhalation Hazards: Avoid prolonged or repeated inhalation of vapors. Use appropriate respiratory protection in case of potential exposure.
-
Skin and Eye Contact: Avoid direct skin and eye contact. Wear appropriate personal protective equipment (PPE), including gloves and eye protection.
-
Storage: Store in a cool, dry, and well-ventilated area away from incompatible materials.
Frequently Asked Questions (FAQ)
Q: Is 4-ethyl-2,2-dimethyloctane toxic?
A: While not acutely toxic in the same way as some other chemicals, prolonged or high-level exposure to its vapors can still cause irritation and potential health problems. Appropriate safety precautions should always be followed.
Q: What are the environmental impacts of 4-ethyl-2,2-dimethyloctane?
A: As a hydrocarbon, its release into the environment can contribute to air pollution. Its biodegradability and potential impact on aquatic ecosystems require further investigation.
Q: Can 4-ethyl-2,2-dimethyloctane be synthesized at home?
A: No, synthesizing this compound at home is not recommended due to the complexity of the synthesis process, the need for specialized equipment and chemicals, and the inherent safety risks involved.
Q: Are there any regulatory restrictions on the use of 4-ethyl-2,2-dimethyloctane?
A: Specific regulations will vary depending on the region and intended application. It’s important to consult relevant safety data sheets and regional regulations before use.
Conclusion
4-Ethyl-2,2-dimethyloctane, while perhaps not a household name, is a fascinating example of a branched alkane with unique properties. Its molecular structure, carefully determined physical and chemical characteristics, potential synthetic routes, and possible applications highlight the intricate world of organic chemistry and its industrial relevance. Further research into its applications and environmental impact would be beneficial in determining its role in various industries. Remember to always prioritize safety when handling any chemical, and consult relevant safety data sheets and regulations before use.
Latest Posts
Latest Posts
-
Human Muscles Coloring Answer Key
Sep 15, 2025
-
Diffusion And Osmosis Worksheet Answers
Sep 15, 2025
-
Magnification Of A Compound Microscope
Sep 15, 2025
-
Esf Are Organized Groups Of
Sep 15, 2025
-
Post Test The Romantic Era
Sep 15, 2025
Related Post
Thank you for visiting our website which covers about 4 Ethyl 2 2 Dimethyloctane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.