Is N2 Paramagnetic Or Diamagnetic
khabri
Sep 14, 2025 · 6 min read
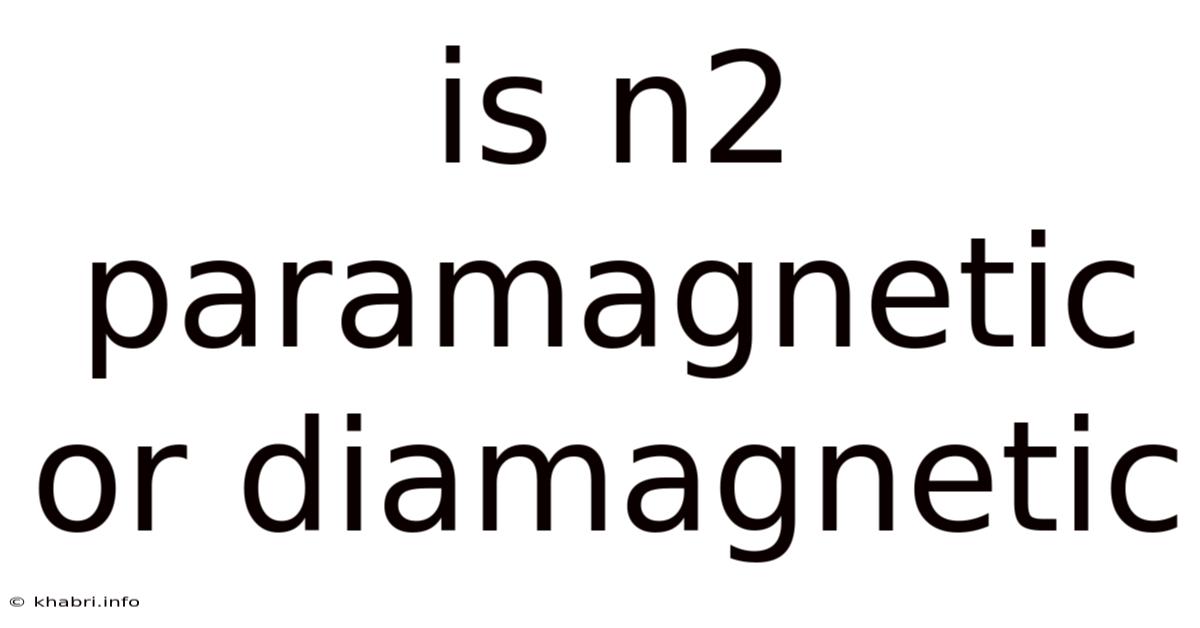
Table of Contents
Is N₂ Paramagnetic or Diamagnetic? Unveiling the Secrets of Nitrogen's Magnetism
Understanding the magnetic properties of molecules is a fundamental concept in chemistry. This article delves into the question: Is N₂ paramagnetic or diamagnetic? We'll explore the underlying principles of magnetism, the electron configuration of nitrogen, and the molecular orbital theory to definitively answer this question. Furthermore, we'll examine the implications of nitrogen's magnetic properties in various applications.
Introduction to Paramagnetism and Diamagnetism
All matter interacts with a magnetic field, although the strength and nature of this interaction vary significantly. Substances can be classified based on their magnetic behavior into three main categories: diamagnetic, paramagnetic, and ferromagnetic.
-
Diamagnetic substances: These materials possess only paired electrons. When exposed to an external magnetic field, they weakly repel the field. This repulsion is due to the slight distortion of the electron orbitals caused by the external field. Diamagnetism is a universal property, present in all matter, but it is often weak and masked by stronger magnetic effects if present.
-
Paramagnetic substances: These materials contain unpaired electrons. Unpaired electrons possess a magnetic moment, and in the presence of an external magnetic field, they align their magnetic moments with the field, resulting in a net attraction. Paramagnetism is generally weaker than ferromagnetism.
-
Ferromagnetic substances: These materials exhibit a strong attraction to magnetic fields due to the alignment of electron spins within domains. This alignment persists even in the absence of an external field, resulting in permanent magnetism. Examples include iron, nickel, and cobalt.
Electronic Configuration of Nitrogen (N)
To determine whether N₂ is paramagnetic or diamagnetic, we need to examine the electronic configuration of a nitrogen atom. Nitrogen has an atomic number of 7, meaning it has 7 electrons. Its electronic configuration is 1s²2s²2p³. Crucially, we see that there are three electrons in the 2p subshell. These electrons occupy three separate 2p orbitals individually, each with one unpaired electron. This is crucial for understanding the magnetic behavior of the nitrogen molecule.
Molecular Orbital Diagram of N₂
Nitrogen exists as a diatomic molecule (N₂). To accurately understand its magnetic properties, we need to consider its molecular orbitals. Using the molecular orbital theory, we can construct a molecular orbital diagram for N₂. The 2s and 2p atomic orbitals of each nitrogen atom combine to form sigma (σ) and pi (π) molecular orbitals.
-
σ2s and σ*2s orbitals: These are formed by the head-on overlap of the 2s atomic orbitals. The σ2s orbital is bonding, while the σ*2s orbital is antibonding.
-
σ2p and σ*2p orbitals: These are formed by the head-on overlap of the 2pz atomic orbitals (where z is the internuclear axis). Again, the σ2p is bonding and σ*2p is antibonding.
-
π2p and π*2p orbitals: These are formed by the side-on overlap of the 2px and 2py atomic orbitals. There are two degenerate π2p bonding orbitals and two degenerate π*2p antibonding orbitals.
In the N₂ molecule, the 14 electrons (7 from each nitrogen atom) fill the molecular orbitals according to the Aufbau principle and Hund's rule. The filling order is: σ2s, σ2s, σ2p, π2p, π2p.
After filling these orbitals, we find that all electrons are paired. The σ2s and σ2s orbitals each contain two electrons. The σ2p orbital contains two electrons. Importantly, both π2p bonding orbitals are filled with two electrons each. The antibonding π2p orbitals remain empty.
This means that all electrons in the N₂ molecule are paired.
Conclusion: N₂ is Diamagnetic
Because all the electrons in the N₂ molecule are paired, there is no net magnetic moment. Consequently, N₂ is diamagnetic. It will exhibit a weak repulsion in the presence of an external magnetic field. The presence of paired electrons effectively cancels out any individual magnetic moments. The molecular orbital diagram clearly shows the absence of unpaired electrons, solidifying the diamagnetic nature of nitrogen gas.
Why Understanding Magnetic Properties Matters
Understanding the magnetic properties of molecules like N₂ is crucial in several scientific fields:
-
Material Science: The magnetic behavior of materials plays a significant role in the development of new technologies. Knowing whether a substance is diamagnetic or paramagnetic allows scientists to design materials with specific magnetic properties for applications like magnetic resonance imaging (MRI), magnetic data storage, and sensors.
-
Spectroscopy: Techniques like electron paramagnetic resonance (EPR) spectroscopy rely on the presence of unpaired electrons. Knowing the magnetic properties of a molecule can help in interpreting EPR spectra.
-
Catalysis: The magnetic properties of catalysts can influence their reactivity and selectivity. Understanding the magnetic behavior of molecules involved in catalytic processes can aid in the design of more efficient catalysts.
-
Atmospheric Chemistry: Nitrogen gas (N₂) is the most abundant component of Earth's atmosphere. Its diamagnetic nature contributes to its overall chemical and physical behavior within the atmosphere.
Frequently Asked Questions (FAQ)
Q1: Can the magnetic properties of a molecule change under different conditions?
A1: While the fundamental magnetic properties of a molecule are determined by its electronic structure, external factors like temperature and pressure can subtly influence the degree of magnetic susceptibility. However, the basic classification (diamagnetic or paramagnetic) generally remains consistent.
Q2: Is it possible for a molecule to be both diamagnetic and paramagnetic?
A2: No, a molecule cannot be simultaneously diamagnetic and paramagnetic. These are mutually exclusive classifications based on the presence or absence of unpaired electrons. A molecule with unpaired electrons is paramagnetic, while one with only paired electrons is diamagnetic.
Q3: How can I experimentally determine if a substance is diamagnetic or paramagnetic?
A3: The simplest method is using a Gouy balance or a similar magnetic susceptibility measurement technique. These methods measure the force exerted on a sample in a strong magnetic field. Diamagnetic substances are slightly repelled, while paramagnetic substances are attracted.
Q4: Are there any exceptions to the rules of paramagnetism and diamagnetism?
A4: While the general principles are well-established, there can be complexities in molecules with extensive conjugation or those exhibiting strong spin-orbit coupling. However, these are exceptions and do not invalidate the core concepts of paramagnetism and diamagnetism.
Conclusion
In summary, through an examination of the electronic configuration of nitrogen and the molecular orbital diagram of N₂, we have conclusively determined that N₂ is diamagnetic. The absence of unpaired electrons leads to a net zero magnetic moment and a weak repulsion in a magnetic field. This fundamental understanding of nitrogen's magnetic behavior is crucial for various scientific applications and provides a solid foundation for further exploration in the realm of molecular magnetism. Understanding the magnetic properties of molecules helps us unravel the secrets of the matter at a fundamental level.
Latest Posts
Latest Posts
-
The Norton Sampler 10th Edition
Sep 14, 2025
-
Function Of The Papillary Muscles
Sep 14, 2025
-
What Helps Bone Resist Compression
Sep 14, 2025
-
Accounting Profit Is Equal To
Sep 14, 2025
-
What Value Would Be Returned
Sep 14, 2025
Related Post
Thank you for visiting our website which covers about Is N2 Paramagnetic Or Diamagnetic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.