Is Alcl3 Polar Or Nonpolar
khabri
Sep 11, 2025 · 5 min read
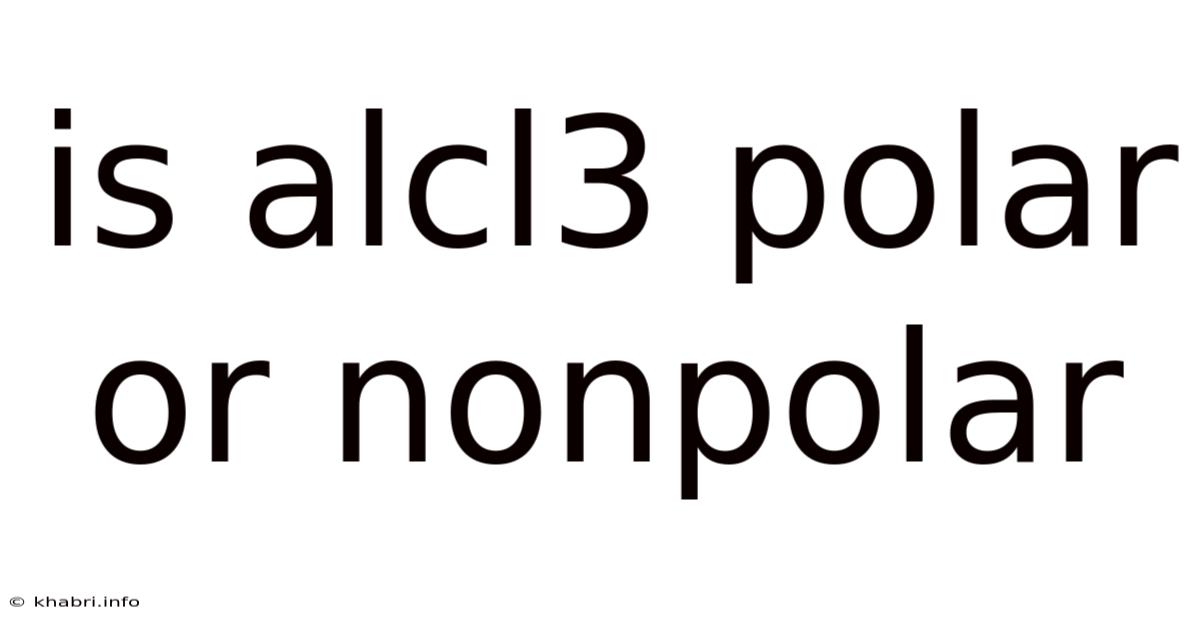
Table of Contents
Is AlCl3 Polar or Nonpolar? A Deep Dive into Molecular Geometry and Polarity
Understanding whether a molecule is polar or nonpolar is crucial in chemistry, impacting its properties and behavior. This article will thoroughly explore the polarity of aluminum chloride (AlCl3), delving into its molecular geometry, bond polarity, and overall dipole moment. We'll unravel the complexities behind this seemingly simple question, providing a comprehensive understanding accessible to both students and enthusiasts.
Introduction: Understanding Polarity
Before diving into AlCl3, let's establish a foundational understanding of molecular polarity. Polarity arises from the unequal sharing of electrons in a covalent bond. This unequal sharing is due to differences in electronegativity – the ability of an atom to attract electrons towards itself in a chemical bond. When atoms with significantly different electronegativities bond, the electrons are drawn more closely to the more electronegative atom, creating a polar bond. This creates a dipole moment, a vector quantity representing the separation of positive and negative charges within the molecule.
A molecule's overall polarity, however, isn't solely determined by individual bond polarities. The molecular geometry – the three-dimensional arrangement of atoms – plays a critical role. If the individual bond dipoles cancel each other out due to symmetry, the molecule is considered nonpolar, even if it contains polar bonds. Conversely, if the bond dipoles don't cancel, the molecule is polar, possessing a net dipole moment.
The Case of AlCl3: Molecular Geometry and Bond Polarity
Aluminum chloride (AlCl3) is a fascinating case study because its polarity depends on its phase – solid, liquid, or gas. Let's examine each phase separately.
Solid State AlCl3: In its solid state, AlCl3 exists as a layered crystal structure. Each aluminum atom is surrounded by six chlorine atoms in an octahedral arrangement. This structure is a result of the strong interactions between the aluminum and chlorine atoms. While individual Al-Cl bonds are polar (chlorine is more electronegative than aluminum), the symmetrical arrangement of these bonds in the solid lattice leads to a cancellation of the dipole moments. Therefore, solid AlCl3 is considered nonpolar.
Gaseous and Liquid State AlCl3: The situation changes dramatically in the gaseous and liquid phases. In these phases, AlCl3 exists as a monomeric molecule with a trigonal planar geometry. This means the aluminum atom is at the center, surrounded by three chlorine atoms arranged in a flat, triangular shape with bond angles of approximately 120°.
Each Al-Cl bond in this monomeric form is polar due to the difference in electronegativity between aluminum (1.61) and chlorine (3.16). However, because of the symmetrical trigonal planar geometry, the three individual bond dipoles are equal in magnitude and point in directions that cancel each other out. Therefore, gaseous and liquid AlCl3 are also considered nonpolar, despite having polar bonds.
This might seem counterintuitive: polar bonds, nonpolar molecule. But it highlights the crucial role of molecular geometry in determining overall polarity. The symmetry of the trigonal planar arrangement perfectly balances the bond dipoles, resulting in a zero net dipole moment.
Further Analysis: Electronegativity and Bond Dipoles
The electronegativity difference between aluminum and chlorine contributes to the polar nature of the individual Al-Cl bonds. While the difference isn't as large as in some other polar bonds (e.g., O-H), it's significant enough to create a dipole in each bond. However, as we’ve seen, the geometry negates the overall polarity.
Comparing AlCl3 with Other Compounds
It's helpful to compare AlCl3 with other compounds to solidify our understanding. For instance, consider boron trichloride (BCl3), which also has a trigonal planar geometry. Similar to AlCl3, BCl3 is nonpolar due to the symmetrical cancellation of bond dipoles despite having polar B-Cl bonds. However, molecules like water (H2O) or ammonia (NH3), with bent and pyramidal geometries respectively, possess significant net dipole moments due to the non-cancellation of polar bond dipoles. These examples underscore the interconnectedness of molecular geometry and polarity.
Experimental Evidence and Applications
The nonpolar nature of gaseous and liquid AlCl3 is supported by experimental observations of its physical properties. For example, its relatively low boiling point reflects weak intermolecular forces, which are typical of nonpolar substances. AlCl3's applications leverage its properties, including its use as a catalyst in organic chemistry reactions. The understanding of its polarity is vital for predicting its reactivity and behavior in these applications.
Frequently Asked Questions (FAQs)
Q: Why does the polarity of AlCl3 change with its phase?
A: The phase change alters the molecular structure of AlCl3. In the solid state, it forms a complex, symmetrical structure where the dipole moments cancel. In the gaseous and liquid states, it exists as a monomer with a trigonal planar geometry, which also leads to cancellation of dipole moments despite the presence of polar bonds.
Q: Could AlCl3 ever become polar under certain conditions?
A: Under extreme conditions, or if it reacted to form a different compound, the geometry could change, possibly leading to a polar molecule. However, in its common forms (solid, liquid, gas), it remains nonpolar.
Q: How does the electronegativity difference affect the Al-Cl bond strength?
A: The electronegativity difference contributes to the bond's polar character but doesn't directly determine its strength. Bond strength is also influenced by factors like the size and effective nuclear charge of the atoms involved.
Q: Are there any exceptions to the rule that symmetrical molecules are nonpolar?
A: Although generally true, there are some exceptions, particularly in cases of molecules with very high symmetry and significant internal charge separation.
Conclusion: The Nonpolar Nature of AlCl3
In conclusion, despite having polar Al-Cl bonds, both gaseous and liquid aluminum chloride (AlCl3) are considered nonpolar due to their symmetrical trigonal planar geometry. The symmetrical arrangement of the bonds leads to a cancellation of individual bond dipoles, resulting in a zero net dipole moment. The solid state exhibits a different structure leading to nonpolarity due to the symmetrical cancellation of dipole moments within the crystal lattice. This detailed analysis emphasizes the crucial interplay between molecular geometry and bond polarity in determining the overall polarity of a molecule, making AlCl3 a prime example of this important chemical concept. Understanding this concept is vital for predicting the chemical and physical properties of various compounds and is crucial for successful application in different fields of chemistry.
Latest Posts
Latest Posts
-
The Watergate Scandal Illustrates The
Sep 12, 2025
-
Understanding In Science Means That
Sep 12, 2025
-
Topic 3 Assessment Form B
Sep 12, 2025
-
Satisficing Is The Tendency Of
Sep 12, 2025
-
Moist Heat Damages Microorganisms By
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Is Alcl3 Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.