Cis 1 Methyl 2 Propylcyclohexane
khabri
Sep 13, 2025 · 8 min read
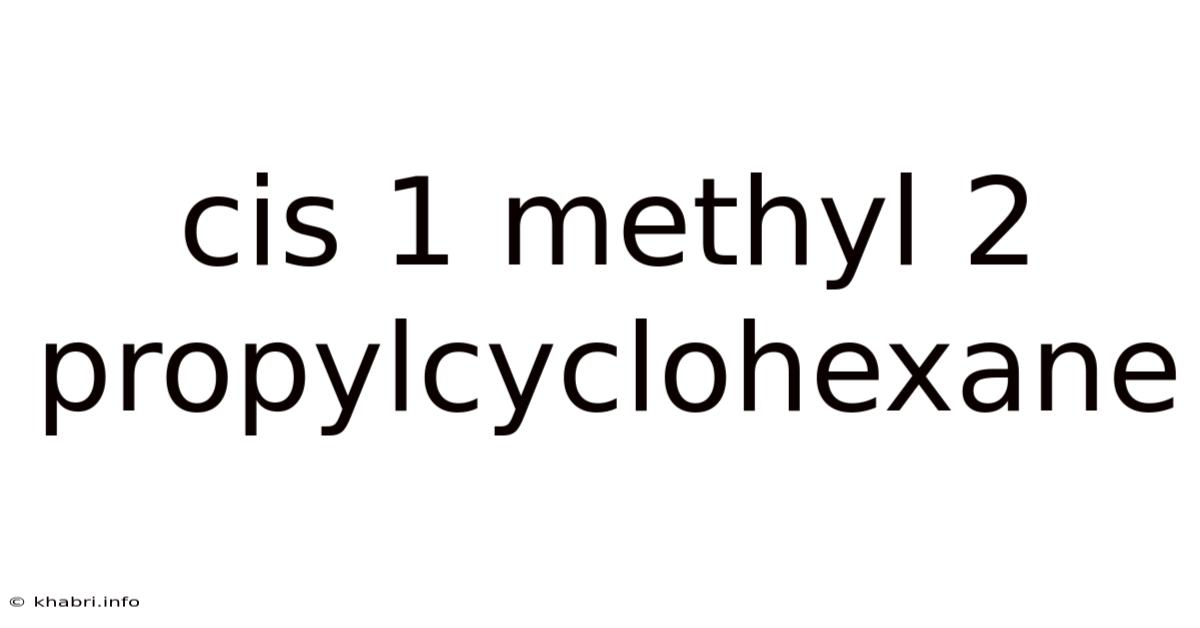
Table of Contents
Delving Deep into cis-1-Methyl-2-propylcyclohexane: Structure, Properties, and Synthesis
Understanding organic molecules, particularly those with complex structures like cis-1-methyl-2-propylcyclohexane, requires a detailed examination of their properties and behavior. This comprehensive article aims to provide a thorough understanding of this specific molecule, covering its structure, stereochemistry, physical and chemical properties, potential synthesis routes, and applications. This exploration will cater to a broad audience, from undergraduate chemistry students to those with a general interest in organic chemistry.
Introduction: Understanding the Molecule
cis-1-methyl-2-propylcyclohexane is a saturated hydrocarbon belonging to the cyclohexane family. The "cyclohexane" part indicates a six-membered carbon ring with single bonds. The "1-methyl-2-propyl" describes the substituents attached to the ring: a methyl group (CH₃) at position 1 and a propyl group (CH₂CH₂CH₃) at position 2. The crucial "cis" prefix specifies the relative spatial arrangement of these substituents. In a cis isomer, both the methyl and propyl groups lie on the same side of the cyclohexane ring. This contrasts with the trans isomer, where they would be on opposite sides. Understanding this stereochemistry is critical because it significantly impacts the molecule's properties. This detailed analysis will clarify the intricacies of its structure, properties, and potential synthesis.
Structural Analysis: Conformations and Stereochemistry
The six-membered cyclohexane ring doesn't exist as a flat structure. To minimize steric strain (repulsion between atoms), it adopts a chair conformation. In this conformation, the carbon atoms are arranged in a three-dimensional structure resembling a chair. Each carbon atom has two substituents: one axial (pointing up or down) and one equatorial (pointing roughly outwards).
In cis-1-methyl-2-propylcyclohexane, the methyl and propyl groups are both on the same side of the ring. This leads to two possible chair conformations: one with both groups axial and another with both groups equatorial. However, the equatorial conformation is significantly more stable due to reduced steric interactions. Having both bulky groups in the axial position causes substantial 1,3-diaxial interactions leading to significant energy destabilization.
The axial positions are closer to other hydrogen atoms on the ring, leading to greater steric hindrance. Equatorial positions provide more space, minimizing these interactions. This conformational preference greatly influences the molecule's overall shape and reactivity. The energy difference between the two conformers can be calculated using various computational methods, allowing for a quantitative understanding of the conformational stability.
Furthermore, the stereochemistry of cis-1-methyl-2-propylcyclohexane prohibits the formation of a meso compound. A meso compound is a molecule with chiral centers but an internal plane of symmetry making the molecule achiral. Because the methyl and propyl groups are on the same side of the ring, there's no such symmetry, resulting in a chiral molecule. Therefore, cis-1-methyl-2-propylcyclohexane exists as a pair of enantiomers.
Key Structural Points:
- Saturated hydrocarbon: Contains only single carbon-carbon bonds.
- Cyclohexane ring: A six-membered ring of carbon atoms.
- Cis isomer: Methyl and propyl groups on the same side of the ring.
- Chair conformation: The preferred three-dimensional structure of the ring.
- Chiral molecule: Possesses no plane of symmetry and exists as enantiomers.
Physical Properties: Boiling Point, Density, and Solubility
The physical properties of cis-1-methyl-2-propylcyclohexane are primarily determined by its size, shape, and intermolecular forces. Like most alkanes, it's a nonpolar molecule, meaning it has a relatively low boiling point compared to molecules with similar molecular weights but containing polar functional groups like alcohols or ketones.
- Boiling Point: The boiling point would be higher than that of simpler cycloalkanes but lower than those with significantly longer alkyl chains due to increased van der Waals forces. Precise measurement would require experimental determination.
- Density: The density would be slightly less than water, typical for nonpolar hydrocarbons. The exact value is again experimentally determined and would depend on temperature.
- Solubility: It's expected to be insoluble in water due to its nonpolar nature. However, it would be soluble in nonpolar organic solvents like hexane or ether because of the "like dissolves like" principle.
The specific values of these properties can be obtained through experimental methods like gas chromatography, mass spectrometry, and various spectroscopic techniques.
Chemical Properties: Reactivity and Reactions
cis-1-methyl-2-propylcyclohexane, as a saturated hydrocarbon, is relatively unreactive under normal conditions. Its primary reactions involve free radical substitution, particularly halogenation.
- Halogenation: Reaction with halogens (e.g., chlorine or bromine) in the presence of light or heat leads to the substitution of hydrogen atoms by halogen atoms. The reaction is not regioselective; multiple products are possible depending on which hydrogen atoms are substituted.
- Combustion: Like all hydrocarbons, it undergoes complete combustion in the presence of sufficient oxygen, producing carbon dioxide and water. Incomplete combustion can lead to the formation of carbon monoxide and soot.
- Oxidation: Strong oxidizing agents can break the carbon-carbon bonds in the cyclohexane ring, leading to the formation of various oxidation products. This reaction is typically complex and produces a mixture of products.
These are fundamental reactions; more complex reactions would require specific catalysts and conditions.
Synthesis Routes: Potential Methods of Preparation
Synthesizing cis-1-methyl-2-propylcyclohexane requires a strategy that ensures the correct stereochemistry (cis). Several methods could be considered, although achieving high yield and selectivity might be challenging. Some possible routes include:
-
Addition of Methyl and Propyl Groups to Cyclohexene: This would involve sequential addition reactions, potentially using Grignard reagents or other organometallic compounds. Controlling the stereochemistry (cis) during these additions would be the main challenge. Careful choice of reagents and reaction conditions would be crucial.
-
Reduction of a Suitable Cyclohexene Derivative: Starting with a cyclohexene derivative that already possesses the methyl and propyl groups in the desired cis configuration, followed by catalytic hydrogenation (reduction with hydrogen gas and a catalyst like palladium or platinum) would yield the target compound. The synthesis of the cis-substituted cyclohexene would need to be carefully planned.
-
Ring-Closing Metathesis (RCM): This method utilizes transition-metal catalysts (e.g., ruthenium-based catalysts) to form a cyclohexane ring from a diene precursor. The careful design of the diene precursor would ensure the correct placement and stereochemistry of the methyl and propyl groups. This approach offers a potentially more efficient route but requires sophisticated synthetic chemistry.
Choosing the optimal synthesis pathway depends on factors such as reagent availability, cost, yield, and the desired level of purity. Extensive optimization of reaction conditions might be needed for any of these pathways.
Applications and Importance: Potential Uses
While cis-1-methyl-2-propylcyclohexane isn't a widely used industrial chemical with specific applications yet documented in the literature, understanding its properties and synthesis is essential for several reasons:
- Fundamental Organic Chemistry: It serves as an excellent example for studying stereochemistry, conformational analysis, and the impact of molecular structure on properties.
- Model System for Research: Its relatively simple structure, yet demonstrably complex stereochemistry, can be a valuable model system for studying various chemical and physical phenomena.
- Potential in Material Science: As a saturated hydrocarbon, it could potentially find applications in the development of new materials or as a component in fuel mixtures. Further research could explore its utility in specific areas.
Frequently Asked Questions (FAQ)
Q: What is the difference between cis- and trans-1-methyl-2-propylcyclohexane?
A: The difference lies in the relative spatial arrangement of the methyl and propyl groups. In the cis isomer, both groups are on the same side of the ring. In the trans isomer, they are on opposite sides. This difference significantly affects their properties, particularly their conformational stability and physical properties.
Q: Is cis-1-methyl-2-propylcyclohexane optically active?
A: Yes, it is optically active because it is a chiral molecule; it lacks a plane of symmetry and exists as a pair of enantiomers (mirror images).
Q: How can I determine the stereochemistry experimentally?
A: Techniques such as nuclear magnetic resonance (NMR) spectroscopy, particularly 1H NMR, would allow for the determination of the relative positions of the substituents. The coupling constants and chemical shifts would provide crucial information. X-ray crystallography would offer definitive structural confirmation.
Q: What are the potential hazards associated with handling cis-1-methyl-2-propylcyclohexane?
A: As with most organic solvents, it's important to handle it in a well-ventilated area, avoiding direct skin contact and inhalation of vapors. Specific hazards would need to be determined through proper safety data sheet (SDS) consultation.
Conclusion: A Deeper Understanding
cis-1-methyl-2-propylcyclohexane, despite its seemingly simple structure, presents a rich case study for understanding fundamental principles in organic chemistry. Its stereochemistry, conformational analysis, and potential synthesis routes provide valuable insights into the relationship between molecular structure and properties. While currently lacking widespread applications, its properties make it a potentially valuable model system for further research and development in various fields. This detailed exploration hopefully provides a solid foundation for further investigation into this molecule's intriguing characteristics. Further research into its synthesis, properties, and potential applications is encouraged to expand our understanding of its role in chemistry and related fields.
Latest Posts
Latest Posts
-
Physical And Antimicrobial Agents Crossword
Sep 13, 2025
-
What Is Vertical Marketing System
Sep 13, 2025
-
Working With Three Dimensional Media Enhances
Sep 13, 2025
-
Principles Of Macroeconomics 9th Edition
Sep 13, 2025
-
Mla Citation For The Bible
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Cis 1 Methyl 2 Propylcyclohexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.