4 Ethyl 1 2 Dimethylcyclohexane
khabri
Sep 16, 2025 · 6 min read
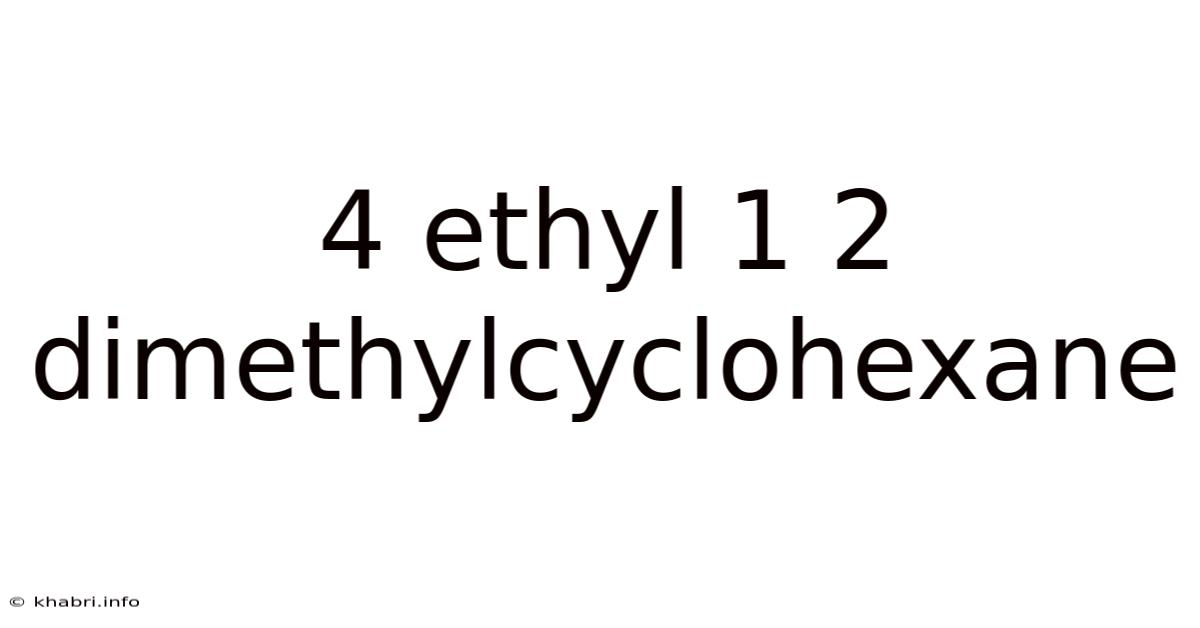
Table of Contents
Exploring 4-Ethyl-1,2-Dimethylcyclohexane: Isomers, Properties, and Significance
4-Ethyl-1,2-dimethylcyclohexane is a fascinating organic molecule, a specific isomer within the broader family of substituted cyclohexanes. Understanding its structure, properties, and potential applications requires delving into the world of stereochemistry and conformational analysis. This comprehensive article will explore the intricacies of this compound, providing a detailed overview for students and researchers alike. This exploration will cover its isomers, its physical and chemical properties, its potential applications (if any), and frequently asked questions regarding its synthesis and characterization.
Introduction to 4-Ethyl-1,2-dimethylcyclohexane
4-Ethyl-1,2-dimethylcyclohexane, as its name suggests, is a cyclohexane ring substituted with three alkyl groups: an ethyl group (–CH₂CH₃) and two methyl groups (–CH₃). The numbering indicates the positions of these substituents on the cyclohexane ring. The '4' denotes the position of the ethyl group, while '1' and '2' indicate the positions of the methyl groups. This seemingly simple structure, however, gives rise to a complex array of isomers due to the possibility of different spatial arrangements of these substituents. Understanding these isomers is crucial to comprehending the molecule's overall properties and behavior.
Isomers of 4-Ethyl-1,2-Dimethylcyclohexane: A World of Stereoisomers
The existence of multiple isomers stems from the chirality of the molecule. The presence of multiple chiral centers (carbon atoms bonded to four different groups) leads to numerous stereoisomers, specifically diastereomers and enantiomers. Let's break it down:
-
Constitutional Isomers: While the name specifies 4-Ethyl-1,2-dimethylcyclohexane, there are several constitutional isomers possible. The ethyl and methyl groups could occupy different positions on the cyclohexane ring, altering the chemical formula but maintaining the overall composition (C₁₀H₂₀).
-
Stereoisomers: Focusing specifically on 4-Ethyl-1,2-dimethylcyclohexane, the major source of isomerism comes from the spatial arrangement of the substituents. The cyclohexane ring itself can exist in two conformations: chair and boat. The chair conformation is significantly more stable due to reduced steric hindrance.
-
Chair Conformations: Even within the chair conformation, various arrangements are possible. The ethyl and methyl groups can be either axial (pointing up or down perpendicular to the ring) or equatorial (pointing outwards, roughly parallel to the plane of the ring). The different combinations of axial and equatorial arrangements lead to multiple diastereomers. These diastereomers have different physical properties such as melting point and boiling point. Furthermore, some of these diastereomers may have chiral centers and exist as enantiomers.
-
Enantiomers: Enantiomers are non-superimposable mirror images of each other. If the molecule contains a chiral center (a carbon atom with four different groups attached), then it exists as a pair of enantiomers. In this case, certain chair conformations will exhibit chirality.
-
Identifying and characterizing these isomers necessitates advanced techniques like nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography. NMR spectroscopy, particularly ¹H NMR and ¹³C NMR, is incredibly useful in differentiating between the various isomers based on chemical shifts and coupling patterns.
Physical and Chemical Properties
Determining the precise physical properties (such as melting point, boiling point, density, and refractive index) of 4-ethyl-1,2-dimethylcyclohexane necessitates knowing the specific isomer under consideration. Each isomer possesses unique physical properties due to subtle differences in their molecular geometry and intermolecular forces. Generally speaking, we expect the following:
-
Non-polar Nature: Being composed primarily of carbon and hydrogen atoms, 4-ethyl-1,2-dimethylcyclohexane is a non-polar molecule. This results in low solubility in polar solvents like water but high solubility in non-polar organic solvents like hexane or benzene.
-
Volatility: Its relatively low molecular weight contributes to some degree of volatility. The exact boiling point will vary depending on the specific isomer.
-
Flammability: As a hydrocarbon, it is flammable and should be handled with care away from open flames and ignition sources.
-
Chemical Reactivity: The chemical reactivity is relatively low due to the saturated nature of the cyclohexane ring and the alkyl substituents. It is relatively inert to most common reagents under ordinary conditions. However, under specific reaction conditions (such as high temperature or presence of strong oxidizing agents), it could undergo reactions like combustion or oxidation.
Potential Applications (if any) and Further Research
Currently, there isn't widespread industrial or commercial application specifically documented for 4-ethyl-1,2-dimethylcyclohexane. This is partly due to the challenges in isolating specific isomers and the relatively low reactivity of the molecule. However, its structural similarity to other cyclohexane derivatives suggests potential applications that warrant further exploration.
Further research could investigate its:
- Use as a solvent: Due to its non-polar nature, it could potentially find use as a solvent in specific organic synthesis reactions or in other industrial processes.
- Potential in polymer synthesis: It could be used as a building block or a modifier in the synthesis of specific polymers, potentially influencing the final polymer's properties.
- Role as a model compound: Its structure makes it suitable as a model compound for studying conformational analysis and the effect of steric interactions on molecular properties.
Synthesis and Characterization
Synthesizing 4-ethyl-1,2-dimethylcyclohexane would likely involve multi-step procedures, potentially beginning with appropriately substituted cyclohexenes. Specific reaction pathways would depend heavily on the desired isomer. Grignard reactions, alkylation reactions, or other organic reactions might be employed, but optimizing the synthesis to obtain a specific isomer would require careful reaction design and control.
Characterization involves sophisticated techniques such as:
- Gas Chromatography (GC): Useful for separation and identification of different isomers based on their boiling points.
- Gas Chromatography-Mass Spectrometry (GC-MS): Provides both separation and structural information.
- Nuclear Magnetic Resonance (NMR) Spectroscopy (¹H and ¹³C): Crucial for determining the exact structure and confirming the presence of specific isomers.
- X-ray Crystallography: Can provide highly accurate 3D structural information if a crystalline sample of the specific isomer can be obtained.
Frequently Asked Questions (FAQ)
Q1: What is the difference between 4-ethyl-1,2-dimethylcyclohexane and its isomers?
A1: The difference lies in the spatial arrangement of the ethyl and methyl groups on the cyclohexane ring. Different arrangements lead to various stereoisomers (diastereomers and enantiomers), each with unique physical and potentially chemical properties.
Q2: How many stereoisomers are possible for 4-ethyl-1,2-dimethylcyclohexane?
A2: The exact number of stereoisomers depends on the consideration of chair and boat conformations and the possibilities of axial vs. equatorial orientations. A detailed conformational analysis is needed for a definitive answer. There are multiple diastereomers, and some of those will exist as enantiomeric pairs.
Q3: Is 4-ethyl-1,2-dimethylcyclohexane chiral?
A3: It depends on the specific isomer. Some of the possible conformations will possess chiral centers, making them chiral molecules.
Q4: What are the main challenges in synthesizing and characterizing 4-ethyl-1,2-dimethylcyclohexane?
A4: The main challenges lie in controlling the stereochemistry during the synthesis to obtain a specific isomer and then definitively characterizing the obtained isomer using advanced spectroscopic techniques to differentiate it from other possible isomers.
Q5: What are the environmental impacts of 4-ethyl-1,2-dimethylcyclohexane?
A5: As a hydrocarbon, it's likely to contribute to greenhouse gas emissions upon combustion. Further research is needed to understand any other potential environmental impacts.
Conclusion
4-Ethyl-1,2-dimethylcyclohexane, while not currently widely applied, presents a rich area for study in stereochemistry and conformational analysis. Its various isomers, each with unique properties, offer opportunities for further investigation into its potential applications and a deeper understanding of its chemical behavior. Further research in synthesis, characterization, and potential applications would greatly contribute to expanding our knowledge of this organic molecule. The complexity arising from relatively simple structural changes emphasizes the importance of meticulous characterization techniques and the significant role of stereochemistry in determining the properties and potential uses of organic compounds.
Latest Posts
Latest Posts
-
Rectangular Prism And Rectangular Pyramid
Sep 16, 2025
-
Takes Place Of A Noun
Sep 16, 2025
-
Timothy Lee Head To Toe
Sep 16, 2025
-
The Well Managed Healthcare Organization
Sep 16, 2025
-
New York Snow Load Map
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about 4 Ethyl 1 2 Dimethylcyclohexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.