3s 4s 3 4 Dimethylhexane
khabri
Sep 11, 2025 · 7 min read
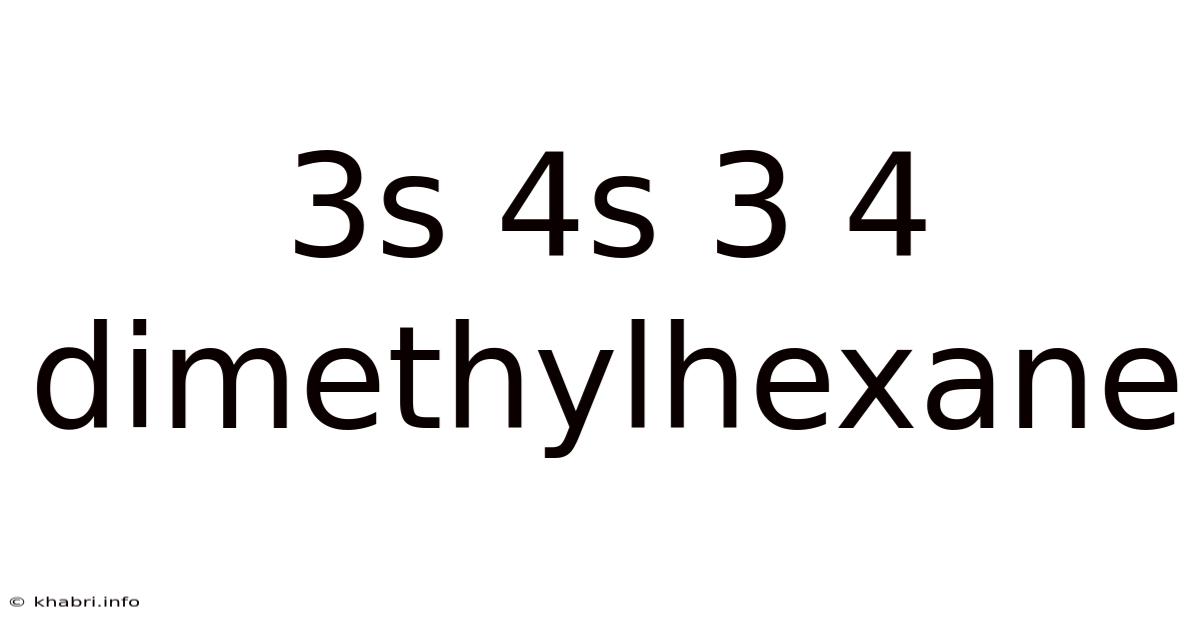
Table of Contents
Decoding the Mystery of 3S, 4S, 3,4-Dimethylhexane: A Deep Dive into Stereochemistry
Understanding the nuances of organic chemistry can be challenging, especially when dealing with complex molecules like 3S, 4S, 3,4-dimethylhexane. This seemingly simple alkane holds a wealth of information about stereochemistry, a crucial concept in understanding the three-dimensional structure and properties of molecules. This article will provide a comprehensive exploration of 3S, 4S, 3,4-dimethylhexane, covering its structure, nomenclature, stereochemistry, and properties, making it accessible to both students and those seeking a deeper understanding of organic chemistry.
Introduction: Understanding Alkanes and Stereochemistry
Before delving into the specifics of 3S, 4S, 3,4-dimethylhexane, let's establish a foundational understanding of alkanes and stereochemistry. Alkanes are saturated hydrocarbons, meaning they consist solely of carbon and hydrogen atoms bonded together with single bonds. They are the simplest type of organic molecule, forming the basis for much more complex structures.
Stereochemistry, on the other hand, is the study of the three-dimensional arrangement of atoms within a molecule and how this arrangement affects its chemical and physical properties. It's crucial because molecules with the same chemical formula (same number and type of atoms) can exist in different three-dimensional forms, called isomers. These isomers can have vastly different properties, highlighting the importance of stereochemical understanding. One key aspect of stereochemistry is chirality, which involves molecules that are non-superimposable mirror images of each other – like your left and right hand. These are called enantiomers. Another important concept is diastereomers, which are stereoisomers that are not mirror images of each other.
Nomenclature and Structure of 3S, 4S, 3,4-Dimethylhexane
The name 3S, 4S, 3,4-dimethylhexane reveals key structural information. Let's break it down:
- Hexane: This indicates a six-carbon chain as the parent alkane.
- 3,4-Dimethyl: This tells us there are two methyl groups (–CH₃) attached to carbons 3 and 4 of the hexane chain.
- 3S, 4S: These prefixes denote the stereochemistry at carbons 3 and 4. The "S" configuration indicates a specific spatial arrangement of the substituents around these chiral carbons, as defined by the Cahn-Ingold-Prelog (CIP) priority rules.
To visualize the structure, imagine a six-carbon chain. On the third carbon, we have a methyl group, and on the fourth carbon, we also have a methyl group. The "S" designation for both carbons indicates a specific three-dimensional arrangement. To determine the "S" configuration, we apply the CIP rules, which prioritize substituents based on atomic number. The higher the atomic number, the higher the priority. For carbon 3, we compare the priorities of the methyl group, the ethyl group (the remaining part of the hexane chain), and the hydrogen. Similarly, we do the same for carbon 4. Following the rules, the specific arrangement of these groups leads to an "S" configuration at both carbons.
Determining the Stereochemistry: Applying the Cahn-Ingold-Prelog (CIP) Rules
The CIP rules are fundamental to assigning absolute configurations (R or S) to chiral centers. Here's a simplified explanation of how they apply to 3S, 4S, 3,4-dimethylhexane:
-
Assign Priorities: For each chiral center (C3 and C4), assign priorities to the four substituents based on atomic number. Higher atomic number gets higher priority. For example, at C3, we would prioritize the ethyl group (C2H5) over the methyl group (CH3) and hydrogen (H).
-
Orient the Molecule: Manipulate the molecule mentally (or on paper) so that the lowest priority group (usually hydrogen) points away from you.
-
Trace the Remaining Groups: Trace the path from the highest priority group (1) to the second-highest priority group (2), then to the third-highest priority group (3).
-
Determine R or S: If the path is clockwise, the configuration is R (rectus, Latin for "right"); if it's counterclockwise, the configuration is S (sinister, Latin for "left").
Applying this process to both C3 and C4 in 3S, 4S, 3,4-dimethylhexane, we arrive at the S configuration for both chiral centers.
Isomers of 3,4-Dimethylhexane: A World of Possibilities
3,4-Dimethylhexane possesses two chiral centers, meaning it can have multiple stereoisomers. Let’s explore the possibilities:
-
Enantiomers: A pair of enantiomers are non-superimposable mirror images. 3S, 4S, 3,4-dimethylhexane has one enantiomer: 3R, 4R, 3,4-dimethylhexane. These two molecules have identical physical properties (except for their interaction with plane-polarized light) but differ in their interaction with other chiral molecules.
-
Diastereomers: Diastereomers are stereoisomers that are not mirror images. In the case of 3,4-dimethylhexane, we can also have 3R, 4S, 3,4-dimethylhexane and 3S, 4R, 3,4-dimethylhexane. These are diastereomers to both 3S,4S and 3R,4R. They have different physical and chemical properties compared to their enantiomers and to each other.
This highlights the importance of understanding stereochemistry. The different stereoisomers of 3,4-dimethylhexane will exhibit varying properties, potentially impacting their applications and interactions in chemical reactions.
Physical and Chemical Properties: A Comparative Analysis
While the exact values may vary slightly depending on experimental conditions, certain trends in the physical and chemical properties can be predicted based on the molecule's structure and stereochemistry.
-
Boiling Point: The boiling point of 3S, 4S, 3,4-dimethylhexane will be similar to its stereoisomers (3R, 4R, 3R, 4S, 3S, 4R) because they have the same molecular weight and similar intermolecular forces.
-
Melting Point: Melting points are more sensitive to subtle differences in molecular packing, so slight variations could be observed among the stereoisomers. The exact values require experimental determination.
-
Solubility: Like other alkanes, 3S, 4S, 3,4-dimethylhexane will be largely insoluble in polar solvents (like water) but soluble in nonpolar solvents (like hexane or benzene). This is due to the nonpolar nature of its carbon-hydrogen bonds.
-
Reactivity: The reactivity of 3S, 4S, 3,4-dimethylhexane will primarily be dictated by the presence of its saturated carbon-carbon and carbon-hydrogen bonds. It will be relatively unreactive under normal conditions, participating primarily in reactions such as combustion and halogenation. However, the stereochemistry might influence the rate and selectivity of some reactions.
Applications and Significance
While 3S, 4S, 3,4-dimethylhexane itself may not have widespread individual applications in industry, its study is crucial for understanding broader principles in organic chemistry. Understanding its stereochemistry is essential for:
-
Drug Design and Development: Many pharmaceutical drugs are chiral molecules, and the specific stereoisomer often determines the drug's efficacy and safety. Understanding how stereochemistry affects biological activity is critical in drug discovery.
-
Polymer Chemistry: The stereochemistry of monomers plays a crucial role in determining the properties of the resulting polymers.
-
Material Science: The design and synthesis of new materials often involves considerations of molecular structure and stereochemistry to tailor properties like strength, flexibility, and reactivity.
Frequently Asked Questions (FAQ)
Q1: What is the difference between 3R, 4R, 3,4-dimethylhexane and 3S, 4S, 3,4-dimethylhexane?
A1: These are enantiomers – non-superimposable mirror images. They have identical physical properties (except for optical activity) but may differ in their biological activity and interactions with other chiral molecules.
Q2: How can I visually represent the different stereoisomers of 3,4-dimethylhexane?
A2: You can use molecular modeling software or draw perspective formulas (wedge-dash representations) to visualize the spatial arrangements of atoms around the chiral centers. Practice drawing these structures to reinforce your understanding.
Q3: Are there other isomers of 3,4-dimethylhexane besides the stereoisomers?
A3: Yes. Constitutional isomers also exist, which possess the same molecular formula but a different connectivity of atoms. For example, 2,3-dimethylhexane is a constitutional isomer of 3,4-dimethylhexane.
Q4: What techniques are used to determine the absolute configuration (R or S) of a molecule experimentally?
A4: Experimental techniques such as X-ray crystallography, and various spectroscopic methods (like NMR) can be used to determine the absolute configuration of a chiral molecule.
Conclusion: The Importance of Precision in Organic Chemistry
3S, 4S, 3,4-dimethylhexane serves as a perfect example of how seemingly minor differences in molecular structure, specifically stereochemistry, can have significant consequences on a molecule's properties and potential applications. Mastering the concepts of chirality, enantiomers, diastereomers, and the Cahn-Ingold-Prelog rules is essential for anyone studying or working in the field of organic chemistry. This detailed exploration should provide a strong foundation for further study and application of these principles in more complex organic molecules. The ability to accurately predict and understand the properties of molecules based on their structure is fundamental to advancements in various scientific fields.
Latest Posts
Latest Posts
-
Working With Words 10th Edition
Sep 11, 2025
-
150 Gallon Propane Tank Dimensions
Sep 11, 2025
-
What Does This Photograph Show
Sep 11, 2025
-
Amygdala Face Images Test Question
Sep 11, 2025
-
5 3 Application Problem Accounting Answers
Sep 11, 2025
Related Post
Thank you for visiting our website which covers about 3s 4s 3 4 Dimethylhexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.