1 Methyl 1 3 Cyclopentanediol
khabri
Sep 05, 2025 · 6 min read
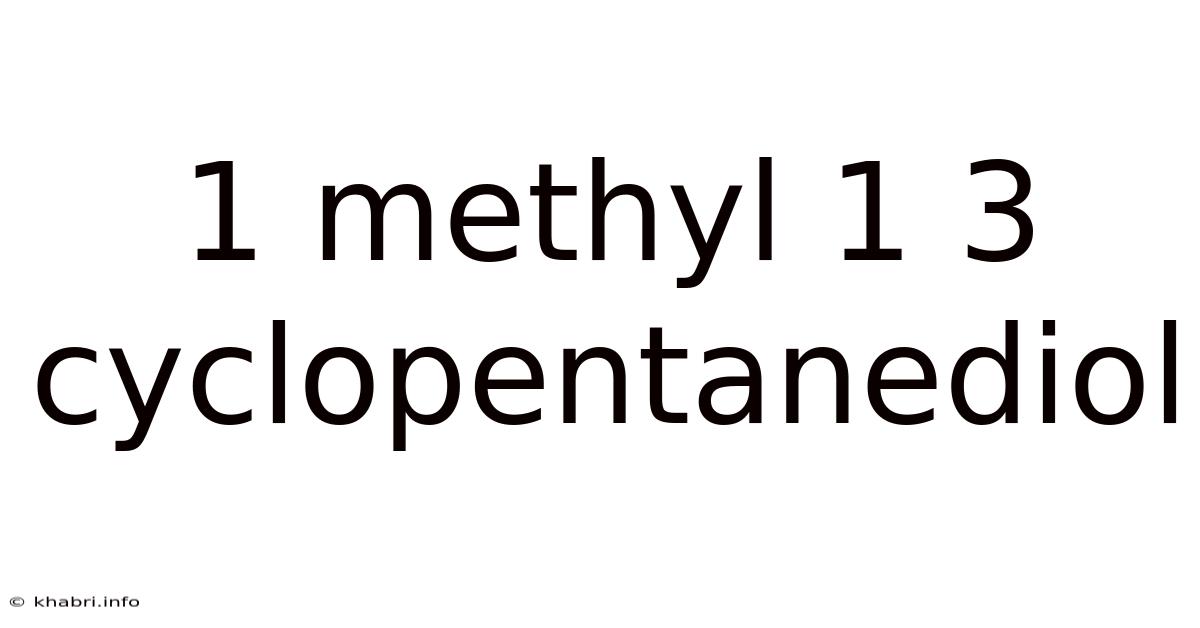
Table of Contents
Unveiling the Mysteries of 1-Methyl-1,3-cyclopentanediol: A Deep Dive
1-Methyl-1,3-cyclopentanediol, often abbreviated as MCPdiol, is a fascinating organic compound with a deceptively simple structure. Its seemingly unassuming chemical formula, C<sub>6</sub>H<sub>12</sub>O<sub>2</sub>, belies a rich chemistry and a growing list of potential applications. This article will delve into the properties, synthesis, applications, and safety aspects of MCPdiol, providing a comprehensive understanding of this intriguing molecule. We'll explore its unique characteristics, examining its structure-activity relationships and potential future roles in various fields.
Understanding the Structure and Properties of MCPdiol
MCPdiol, as its name suggests, is a cyclic diol. The core structure is a five-membered cyclopentane ring, with a methyl group (–CH<sub>3</sub>) and two hydroxyl groups (–OH) attached to the ring. The specific arrangement of these substituents is crucial, influencing its reactivity and properties. The presence of two hydroxyl groups grants it significant polarity, making it soluble in polar solvents like water and alcohols. This polarity is a key factor influencing its potential applications in various chemical processes.
The cis and trans isomers of MCPdiol are possible, resulting from the different spatial arrangements of the hydroxyl groups relative to the cyclopentane ring plane. These isomers exhibit different physical and chemical properties, influencing their interactions with other molecules and their suitability for specific applications. The cis isomer, for instance, might exhibit stronger hydrogen bonding capabilities compared to its trans counterpart due to the proximity of the hydroxyl groups. Understanding these isomeric differences is crucial for optimizing its use in various contexts.
The relatively compact structure of MCPdiol contributes to its stability under various conditions. However, its reactivity is governed by the presence of the hydroxyl groups, which can participate in various chemical reactions, including esterification, etherification, and oxidation. These reactions form the basis of its synthetic utility, allowing for the creation of a wide range of derivatives with diverse properties.
Synthesis of 1-Methyl-1,3-cyclopentanediol: Routes and Challenges
The synthesis of MCPdiol can be approached through several pathways, each presenting its own advantages and disadvantages. One common approach involves the dihydroxylation of 1-methylcyclopentene. This reaction, often facilitated by catalysts like osmium tetroxide (OsO<sub>4</sub>), adds two hydroxyl groups across the double bond of the cyclopentene ring, yielding MCPdiol. While effective, this method often requires careful control of reaction conditions to maximize yield and minimize the formation of byproducts.
Another route involves the epoxidation of 1-methylcyclopentene, followed by ring opening of the epoxide with a suitable reagent. This method provides more flexibility in controlling the stereochemistry of the product, allowing for preferential synthesis of either the cis or trans isomer. However, the choice of reagents and reaction conditions needs to be carefully optimized to achieve high selectivity and yield.
The development of efficient and environmentally friendly synthetic routes for MCPdiol remains an area of active research. Efforts are focused on minimizing the use of hazardous reagents, improving reaction yields, and simplifying the synthetic procedures. Green chemistry principles are being applied to develop sustainable and economically viable methods for the large-scale production of MCPdiol.
Applications of 1-Methyl-1,3-cyclopentanediol: A Diverse Landscape
The unique structural features of MCPdiol, coupled with its reactive hydroxyl groups, open up a wide range of potential applications. Its versatility is reflected in its use as an intermediate in the synthesis of various valuable compounds and its potential applications in diverse fields.
1. Pharmaceutical and Medicinal Chemistry: MCPdiol has emerged as a promising building block in the synthesis of numerous pharmaceuticals and bioactive molecules. Its hydroxyl groups can be readily modified to introduce various functional groups, leading to compounds with tailored properties. For instance, it might serve as a precursor in the synthesis of drugs targeting specific receptors or enzymes. Its potential in drug development is significant, given its relatively non-toxic nature and the ease with which its structure can be modified. Research is ongoing to explore its application in creating novel therapeutic agents.
2. Polymer Chemistry and Materials Science: MCPdiol can be incorporated into polymer chains, imparting unique properties to the resulting materials. Its hydroxyl groups allow for the formation of ester, ether, and urethane linkages, creating polymers with varied flexibility, strength, and thermal stability. These polymers may find applications in diverse fields, including coatings, adhesives, and biomedical implants. The research into MCPdiol-based polymers is expanding, with focus on tailoring the polymer properties for specific applications.
3. Cosmetics and Personal Care: Due to its relatively low toxicity and potential moisturizing properties, MCPdiol has attracted interest in the cosmetics and personal care industry. It might be incorporated into lotions, creams, and other personal care products to enhance their moisturizing effects or to serve as a carrier for other active ingredients. The biocompatibility and skin-friendly nature of MCPdiol are being investigated to ascertain its suitability for this application.
4. Flavor and Fragrance Chemistry: MCPdiol or its derivatives could potentially be used as fragrance components or flavor enhancers. The introduction of various functional groups could significantly alter its olfactory or gustatory properties. The development of MCPdiol-based flavor and fragrance molecules requires careful consideration of their sensory properties and safety profiles.
Safety and Environmental Considerations
While MCPdiol is generally considered to be of low toxicity, proper handling and safety precautions are still necessary. Standard laboratory practices should be followed when working with MCPdiol, including the use of appropriate personal protective equipment (PPE), such as gloves and eye protection. Inhalation or ingestion should be avoided, and any spills should be handled according to established safety protocols. The environmental impact of MCPdiol and its derivatives should also be considered, particularly during large-scale production and disposal. Sustainable synthesis routes and responsible waste management practices are essential to minimize environmental harm.
Frequently Asked Questions (FAQ)
Q1: What is the melting point of MCPdiol?
A1: The melting point of MCPdiol varies slightly depending on the isomeric form (cis or trans). Precise values can be found in specialized chemical databases.
Q2: Is MCPdiol soluble in water?
A2: Yes, MCPdiol is soluble in water due to the presence of its polar hydroxyl groups.
Q3: What are the main challenges in synthesizing MCPdiol?
A3: Challenges include optimizing reaction conditions for high yield and selectivity, minimizing byproduct formation, and developing environmentally friendly synthetic routes.
Q4: What are the potential health effects of exposure to MCPdiol?
A4: While generally considered low toxicity, exposure should be minimized, and standard lab safety procedures should be followed.
Q5: Are there any regulatory restrictions on the use of MCPdiol?
A5: Regulations vary depending on the intended application and geographical location. It is essential to consult relevant regulatory authorities before using MCPdiol in any commercial product or process.
Conclusion: A Molecule with Potential
1-Methyl-1,3-cyclopentanediol represents a fascinating example of a relatively simple molecule with a broad range of potential applications. Its unique structural features and reactive hydroxyl groups make it a versatile building block in various chemical processes. From pharmaceutical development to material science and beyond, MCPdiol promises a diverse future in a multitude of industries. Continued research into its synthesis, properties, and applications will undoubtedly unlock even more of its potential, shaping its role in scientific advancements and technological innovations for years to come. The exploration of sustainable synthetic methods and a deeper understanding of its biological interactions are key to unlocking the full potential of this intriguing molecule.
Latest Posts
Latest Posts
-
For The Hydrogenation Reaction Shown
Sep 07, 2025
-
Name Of The Compound So3
Sep 07, 2025
-
Correctly Label The Following Levers
Sep 07, 2025
-
Been On A Tear Meaning
Sep 07, 2025
-
Chemical Formula Of Magnesium Sulfide
Sep 07, 2025
Related Post
Thank you for visiting our website which covers about 1 Methyl 1 3 Cyclopentanediol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.