1 Halo 2 3 Dimethylbutane
khabri
Sep 10, 2025 · 7 min read
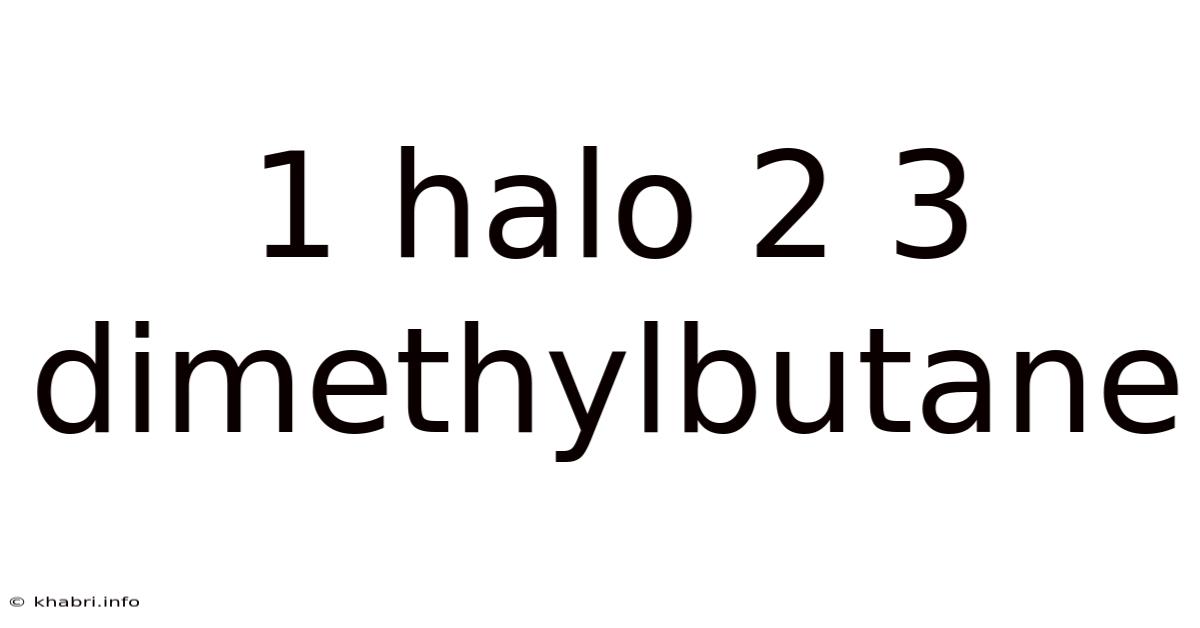
Table of Contents
Unveiling the Secrets of 1-Halo-2,3-Dimethylbutane: A Deep Dive into Structure, Reactivity, and Applications
1-Halo-2,3-dimethylbutane represents a fascinating class of organic compounds, characterized by a branched alkane backbone substituted with a halogen atom at the primary carbon and methyl groups at carbons two and three. Understanding its structure, reactivity, and potential applications requires a detailed examination of its chemical properties and behavior. This comprehensive guide will delve into the intricacies of 1-halo-2,3-dimethylbutane, providing insights relevant to students, researchers, and anyone interested in organic chemistry.
Introduction: Understanding the Basics
Before embarking on a detailed exploration, let's establish a fundamental understanding of 1-halo-2,3-dimethylbutane. The name itself reveals key structural information. The "butane" suffix indicates a four-carbon alkane chain. The "2,3-dimethyl" prefix signifies two methyl (CH₃) groups attached to the second and third carbon atoms. Finally, "1-halo" denotes a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to the first carbon atom. This creates a molecule with a specific arrangement of atoms, influencing its physical and chemical properties. The variations in the halogen atom lead to different isomers, each with unique characteristics. For example, 1-chloro-2,3-dimethylbutane differs from 1-bromo-2,3-dimethylbutane in terms of its boiling point, reactivity, and other properties due to the difference in the halogen atom's electronegativity and atomic size. Understanding this fundamental concept is crucial for appreciating the complexities of this class of compounds. We'll explore these variations in more detail in subsequent sections.
Structural Analysis: Isomers and Conformational Isomers
The structural diversity of 1-halo-2,3-dimethylbutane extends beyond the choice of halogen. While the main structure is defined, the arrangement of atoms in space, particularly the orientation of the methyl groups and the halogen, can lead to different isomers. These isomers are not simply different in name but exhibit distinct properties.
-
Constitutional Isomers: These isomers possess the same molecular formula but differ in the connectivity of their atoms. In the case of 1-halo-2,3-dimethylbutane, constitutional isomers might arise if the halogen atom is placed at a different carbon atom, leading to compounds like 2-halo-2,3-dimethylbutane, which has significantly different reactivity and properties.
-
Stereoisomers: Stereoisomers have the same molecular formula and the same connectivity of atoms but differ in the arrangement of atoms in three-dimensional space. For 1-halo-2,3-dimethylbutane, this is primarily relevant for the chiral center, which is present when the halogen atom is attached at carbon 1 and the two methyl groups are attached at carbons 2 and 3. This lack of symmetry creates an enantiomer with a non-superimposable mirror image. Therefore, 1-halo-2,3-dimethylbutane exhibits optical isomerism, having a pair of enantiomers that rotate plane-polarized light in opposite directions. These enantiomers will have identical chemical properties in achiral environments but may display different interactions with other chiral molecules, as seen in biological systems.
-
Conformational Isomers (Conformers): These are isomers that differ only by rotation around a single bond. Because of the free rotation around single bonds in the molecule, numerous conformers exist for 1-halo-2,3-dimethylbutane. These conformers differ in energy, with some being more stable than others due to steric factors (repulsions between atoms and electron clouds). The most stable conformer will usually be the one where the bulky groups are arranged as far apart as possible to minimize steric hindrance. This contributes to the overall behavior of the molecule, influencing its physical properties such as boiling point and viscosity.
Reactivity: Exploring Chemical Reactions
The reactivity of 1-halo-2,3-dimethylbutane is primarily determined by the presence of the halogen atom and the nature of the carbon-halogen bond. This bond is polar, with the halogen atom being more electronegative than the carbon atom, resulting in a partial positive charge on the carbon and a partial negative charge on the halogen. This polarity makes the carbon-halogen bond susceptible to nucleophilic attack, forming the basis of numerous reactions.
-
Nucleophilic Substitution (SN1 and SN2): The halogen atom can be easily replaced by a nucleophile (an electron-rich species). The mechanism of this substitution can follow either the SN1 (unimolecular nucleophilic substitution) or SN2 (bimolecular nucleophilic substitution) pathway, depending on the steric hindrance around the carbon atom bearing the halogen and the nature of the nucleophile. SN2 reactions are favored with strong nucleophiles and less sterically hindered substrates, while SN1 reactions are favored with weak nucleophiles and more sterically hindered substrates. The products formed will be different depending on the reaction mechanism and the nucleophile involved.
-
Elimination Reactions (E1 and E2): In the presence of a strong base, 1-halo-2,3-dimethylbutane can undergo elimination reactions, resulting in the formation of alkenes. These reactions can follow either the E1 (unimolecular elimination) or E2 (bimolecular elimination) pathway, depending on the reaction conditions and the substrate. E2 reactions are favored with strong bases, while E1 reactions are favored with weaker bases and more sterically hindered substrates. The specific alkene isomers formed will be determined by Zaitsev's rule which favors the formation of the more substituted alkene.
-
Grignard Reagent Formation: Reaction with magnesium in anhydrous ether will form a Grignard reagent, a powerful nucleophile used in various organic syntheses. This Grignard reagent can then react with a variety of electrophiles, opening a pathway for creating more complex organic molecules.
Synthesis: Methods for Preparation
Several synthetic routes can be employed to prepare 1-halo-2,3-dimethylbutane. The specific method employed often depends on the desired halogen and the availability of starting materials. Some common approaches include:
-
Free Radical Halogenation: This method involves the reaction of 2,3-dimethylbutane with a halogen (Cl₂, Br₂) in the presence of UV light. This generates free radicals which can abstract a hydrogen atom from the primary carbon, creating a primary radical that can then react with a halogen molecule to yield 1-halo-2,3-dimethylbutane. However, this method often leads to a mixture of products due to the non-selective nature of free radical reactions.
-
Substitution Reactions: Starting from other functionalized 2,3-dimethylbutane derivatives, substitution reactions can lead to the target compound. For instance, conversion of an alcohol to an alkyl halide via reaction with hydrohalic acids (HCl, HBr, HI) or thionyl chloride (SOCl₂) would produce the desired 1-halo-2,3-dimethylbutane derivative. This method offers greater control over the regioselectivity compared to free radical halogenation.
Applications: Potential Uses in Various Fields
While the specific applications of 1-halo-2,3-dimethylbutane may not be as widely publicized as some other organic compounds, its unique properties make it a potentially valuable compound in several areas:
-
Organic Synthesis Intermediates: Its reactivity, particularly its susceptibility to nucleophilic substitution and elimination reactions, makes 1-halo-2,3-dimethylbutane a valuable intermediate in the synthesis of more complex organic molecules. This opens avenues for developing novel materials and pharmaceuticals.
-
Polymer Synthesis: The possibility of incorporating 1-halo-2,3-dimethylbutane into polymer chains could lead to materials with tailored properties. The halogen atom can introduce specific functionality, influencing the material's physical and chemical properties such as solubility, thermal stability, and reactivity.
-
Solvent: Its relatively non-polar nature, alongside its reactivity profile, makes it a potential candidate for specialized solvent applications in chemical processes.
Frequently Asked Questions (FAQs)
Q: What are the physical properties of 1-halo-2,3-dimethylbutane?
A: The physical properties (melting point, boiling point, density, solubility) vary considerably depending on the specific halogen. Generally, they are volatile liquids at room temperature, with boiling points increasing with the size of the halogen atom. They are generally less dense than water and largely insoluble in water due to their non-polar nature.
Q: Is 1-halo-2,3-dimethylbutane toxic?
A: Like many organic halides, 1-halo-2,3-dimethylbutane can be toxic. The toxicity level varies according to the specific halogen; alkyl bromides and iodides tend to be more toxic than alkyl chlorides. Appropriate safety precautions, including proper handling and disposal, are essential when working with these compounds.
Q: What are the environmental concerns associated with 1-halo-2,3-dimethylbutane?
A: Many organic halides are considered environmental pollutants, and improper disposal can lead to soil and water contamination. Their persistence in the environment and potential bioaccumulation in the food chain are significant concerns.
Conclusion: A Versatile Compound with Unfolding Potential
1-halo-2,3-dimethylbutane, despite its seemingly simple structure, showcases a complex array of structural isomers, reactivity patterns, and potential applications. Its susceptibility to nucleophilic substitutions and eliminations makes it a crucial building block in organic synthesis. Further research into its properties and potential applications could unlock even greater possibilities for this versatile compound in various fields, from materials science to pharmaceutical development. The exploration of its chiral nature and the effects of varying halogens on its reactivity and applications offer rich avenues for future investigation. This deep dive into the world of 1-halo-2,3-dimethylbutane has only scratched the surface; much remains to be discovered and understood about this intriguing molecule.
Latest Posts
Latest Posts
-
7 Normative And Positive Statements
Sep 10, 2025
-
Organic Compounds Alkanes Lab 21
Sep 10, 2025
-
Solubility Of Cacl2 In Water
Sep 10, 2025
-
Chegg Free Shipping Coupon Code
Sep 10, 2025
-
Identify The Highlighted Structure Brain
Sep 10, 2025
Related Post
Thank you for visiting our website which covers about 1 Halo 2 3 Dimethylbutane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.