1 1 Dibromo 2 Chlorocyclopropane
khabri
Sep 09, 2025 · 6 min read
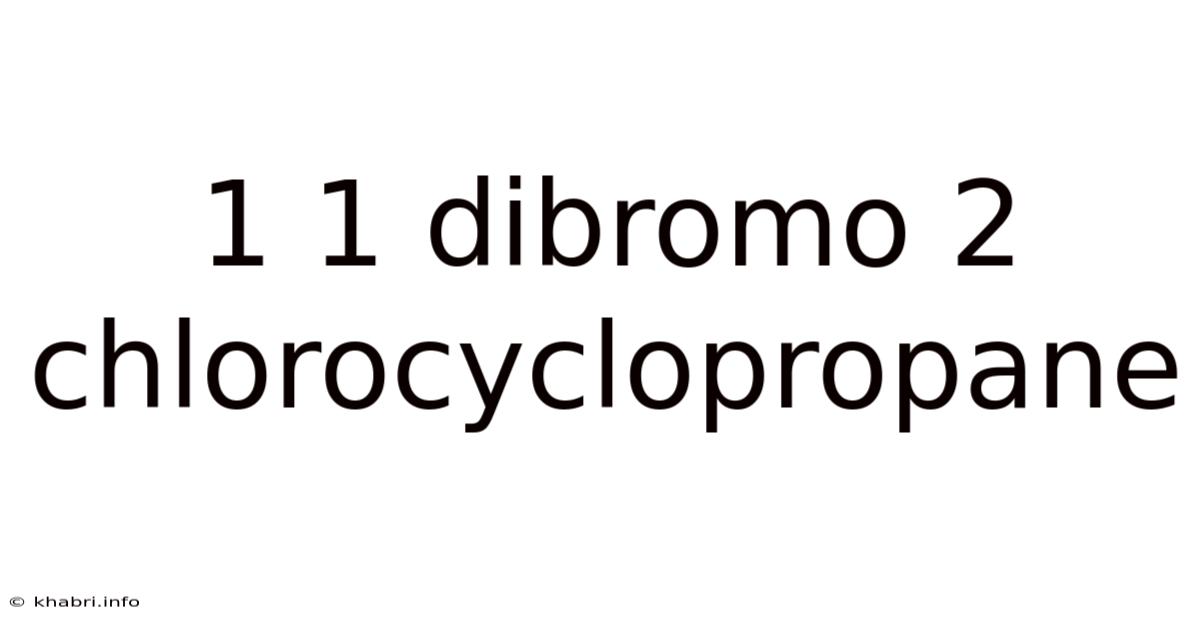
Table of Contents
1,1-Dibromo-2-chlorocyclopropane: A Deep Dive into its Synthesis, Properties, and Potential Applications
1,1-Dibromo-2-chlorocyclopropane, a seemingly simple organic molecule, holds a surprising depth of complexity. This article delves into the fascinating world of this haloalkane, exploring its synthesis, chemical and physical properties, potential applications, and safety considerations. Understanding this compound requires a grasp of organic chemistry principles, but this explanation will be accessible to a broad audience, from undergraduate students to curious enthusiasts.
Introduction: Understanding the Structure and Significance
1,1-Dibromo-2-chlorocyclopropane, often abbreviated as 1,1-dibromo-2-chlorocyclopropane, is a cyclic organic compound characterized by a three-membered carbon ring (cyclopropane) with two bromine atoms attached to one carbon and a chlorine atom attached to another. This specific arrangement of halogens significantly impacts its reactivity and potential applications. While it may not be a household name, understanding its properties is crucial for researchers in fields like organic synthesis, materials science, and potentially, medicinal chemistry. The unique strained ring system of cyclopropanes lends itself to a variety of chemical transformations, making derivatives like 1,1-dibromo-2-chlorocyclopropane valuable building blocks for more complex molecules.
Synthesis of 1,1-Dibromo-2-chlorocyclopropane: A Multifaceted Approach
The synthesis of 1,1-dibromo-2-chlorocyclopropane isn't a trivial undertaking. Several synthetic routes exist, each with its advantages and disadvantages in terms of yield, selectivity, and cost-effectiveness. One common approach involves the reaction of 2-chloropropene with an appropriate brominating agent. The precise conditions, such as the choice of solvent and temperature, significantly influence the success of the reaction and the purity of the final product.
Potential Synthetic Routes:
-
Direct Halogenation of 2-Chloropropene: This approach might involve the use of elemental bromine (Br₂) under carefully controlled conditions. However, this method often leads to a mixture of isomers, requiring purification steps to isolate the desired 1,1-dibromo-2-chlorocyclopropane. Careful control of reaction temperature and the presence of a suitable catalyst can improve selectivity.
-
Addition-Elimination Reactions: This strategy might involve the addition of a brominating agent followed by an elimination reaction to form the cyclopropane ring. This could involve multiple steps and careful reagent selection.
-
Carbene Addition: A potentially more controlled method involves the addition of a dibromocarbene (:CBr₂) to vinyl chloride. Dibromocarbenes can be generated in situ using various methods, offering a degree of control over the reaction pathway and potentially leading to higher yields of the desired product.
Regardless of the chosen synthetic pathway, purification techniques such as distillation, recrystallization, and chromatography are often necessary to obtain a pure sample of 1,1-dibromo-2-chlorocyclopropane. The specific purification method will depend on the scale of the synthesis and the purity requirements.
Physical and Chemical Properties: A Detailed Examination
1,1-Dibromo-2-chlorocyclopropane exhibits several key physical and chemical properties that dictate its behavior and potential applications.
Physical Properties:
-
Appearance: Likely a colorless to pale yellow liquid at room temperature. The exact appearance might vary depending on purity and sample age.
-
Boiling Point: The boiling point would be significantly influenced by the molecular weight and intermolecular forces. Due to the presence of halogens, it would likely have a relatively high boiling point compared to similar non-halogenated cyclopropanes. Precise experimental data would be needed for a definitive value.
-
Density: The density would also be affected by the presence of the heavy bromine atoms, resulting in a density higher than water. Again, precise measurement is required.
-
Solubility: Likely poorly soluble in water due to its nonpolar nature. It might be more soluble in organic solvents such as dichloromethane, diethyl ether, or hexane.
Chemical Properties:
-
Reactivity: The presence of three halogens makes it highly reactive, particularly towards nucleophiles. The strained cyclopropane ring also contributes to its reactivity, making it susceptible to ring-opening reactions.
-
Reactions with Nucleophiles: Nucleophiles such as alkoxides, thiols, and amines can readily attack the electrophilic carbon atoms of the cyclopropane ring, leading to ring opening and the formation of various substituted products.
-
Reactions with Electrophilic Reagents: While less common, reactions with electrophilic reagents are possible, often involving the halogen atoms as reaction sites.
-
Thermal Stability: The stability of the compound under elevated temperatures needs to be carefully investigated. The strained ring and the presence of halogens might make it susceptible to decomposition at higher temperatures.
Potential Applications: Exploring the Possibilities
While 1,1-dibromo-2-chlorocyclopropane is not currently widely used in commercial applications, its unique chemical structure presents intriguing possibilities for future development. Its reactive nature makes it a potentially valuable building block in organic synthesis:
-
Synthesis of complex organic molecules: The presence of the cyclopropane ring and halogens offers diverse opportunities for further functionalization. Ring-opening reactions, halogen substitution, and other transformations could create a wide range of derivatives with diverse properties.
-
Potential in materials science: The compound's chemical properties might be exploitable in materials science. Its potential for polymerization or incorporation into polymer matrices is worth exploring.
-
Pharmaceutical applications: Although speculative, careful investigation into its potential bioactivity could uncover applications in medicinal chemistry. Further research would be needed to assess its toxicity and pharmacological properties.
-
Pesticide precursor: The halogenated nature might be beneficial for applications as a precursor for pesticides, though rigorous testing would be essential to confirm its efficacy and ensure environmental safety.
Safety Considerations: Handling with Care
Handling 1,1-dibromo-2-chlorocyclopropane requires careful attention to safety protocols due to its potential hazards:
-
Toxicity: The compound is likely toxic, and exposure should be minimized. Appropriate personal protective equipment (PPE), including gloves, eye protection, and a laboratory coat, should always be used when handling this chemical. Proper ventilation is also crucial.
-
Reactivity: Its reactivity necessitates handling in inert conditions to prevent unwanted reactions. Contact with strong bases or nucleophiles should be avoided.
-
Environmental Considerations: The release of halogenated compounds into the environment is undesirable. Proper waste disposal procedures must be followed.
Detailed safety data sheets (SDS) should be consulted before any handling or experimental work with this compound.
Frequently Asked Questions (FAQ)
Q: Is 1,1-dibromo-2-chlorocyclopropane commercially available?
A: While not a common laboratory reagent, it is likely that it could be synthesized on demand by specialized chemical suppliers, or custom synthesized by research labs.
Q: What are the major challenges in synthesizing this compound?
A: Challenges include achieving high selectivity to avoid isomer formation, achieving high yields, and dealing with purification complexities.
Q: What are the environmental concerns associated with this compound?
A: Environmental concerns primarily revolve around potential water and soil contamination due to its halogenated nature. Bioaccumulation in living organisms is a possibility that requires investigation.
Q: Are there any known biological activities of this compound?
A: At present, no readily available information exists on its biological activities. This would be a topic for further scientific research.
Conclusion: A Promising Compound for Future Research
1,1-Dibromo-2-chlorocyclopropane, despite its apparent simplicity, is a molecule with a rich potential. Its unique structure and reactivity make it an interesting target for further research in organic synthesis, materials science, and potential medicinal applications. However, its inherent reactivity and potential toxicity necessitate careful handling and thorough safety precautions. Further research is crucial to fully understand its properties and unlock its potential applications while mitigating any associated risks. The synthesis, characterization, and exploration of its chemical reactivity will undoubtedly continue to reveal valuable insights into this fascinating molecule. Continued investigations could establish its role as a useful building block for more complex and potentially valuable molecules, solidifying its place in the world of organic chemistry.
Latest Posts
Latest Posts
-
2 5 9 Chat Bot 2 0
Sep 09, 2025
-
A Nurse Assistant Is Attending
Sep 09, 2025
-
5 Leg Intersection Conflict Points
Sep 09, 2025
-
A Client Defined Sense Of Spirituality
Sep 09, 2025
-
Why Does Facebook Acquire Startups
Sep 09, 2025
Related Post
Thank you for visiting our website which covers about 1 1 Dibromo 2 Chlorocyclopropane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.